
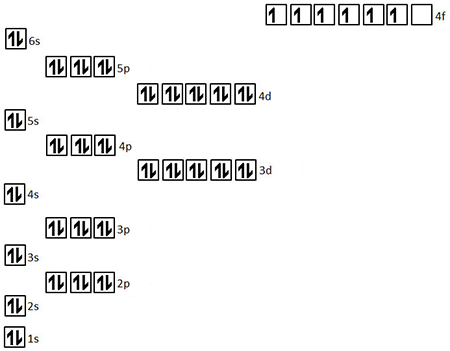
Pauli Exclusion Principle and Hund rule are also put forward to describe the orbitals and electrons in atoms. Schrodinger came up with the idea of having orbitals in an atom. An alternative version of the exclusion principle as applied to atomic electrons states that no two electrons can have the same values of all four. Pauli Exclusion Principle vs Hund Rule After finding the atomic structure, there were so many models to describe how the electrons reside in an atom. An orbital occupied by a pair of electrons of opposite spin is filled: no more electrons may enter it until one of the pair vacates the orbital. Let us examine how the exclusion principle applies to electrons in atoms. The Pauli exclusion principle indicates that, if one of these states is occupied by an electron of spin one-half, the other may be occupied only by an electron of opposite spin, or spin negative one-half. In 1925, the Austrian physicist Wolfgang Pauli (see Figure 13.58) proposed the following rule: No two electrons can have the same set of quantum numbers. (credit: Nobel Foundation, via Wikimedia Commons) He proposed the exclusion principle hypothesized the existence of an important particle, called the neutrino, before it was directly observed made fundamental contributions to several areas of theoretical physics and influenced many students who went on to do important work of their own. As per the Pauli exclusion principle, in a given single atom, no two electrons can have the same or identical set of the four quantum numbers (n,l, ml, and. Le principe dexclusion de Pauli est responsable du fait que la matière ordinaire est stable et occupe un volume. The Pauli exclusion principle states that- no two electrons can have the identical set of quantum numbers or quantum states simultaneously. \): The Austrian physicist Wolfgang Pauli (1900–1958) played a major role in the development of quantum mechanics.


 0 kommentar(er)
0 kommentar(er)
